The Official SecureDocs Blog
Stay on top of the latest tips, trends, and best practices for M&A, fundraising, and other business events.
Browse by topic
Subscribe to our blog
-

M&A
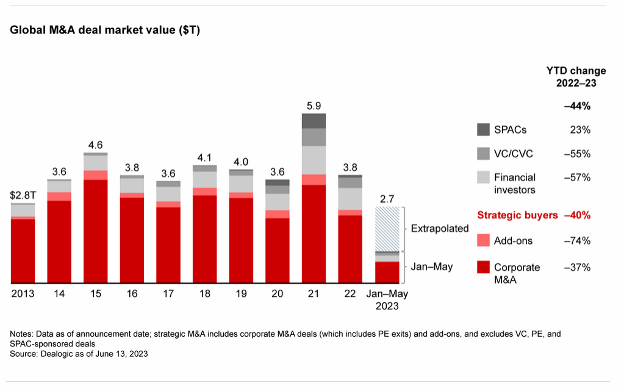
Q3 M&A Recap: Global Activity Sluggish, US Leads
-
Private Equity
Private Equity 2023 Mid-Year Recap: A Tough First Half
-

Virtual Data Room
Revolutionizing Board Communications with Virtual Data Rooms
-

M&A
Q2 2023 Yields Cautious Optimism for M&A
-

M&A
Q1 M&A: Running Against the Wind
-

Life Sciences
Q1 Life Sciences: Resilient in a Changing Landscape
-

IPO
IPOs plummet in 2022. What Comes Next?
-
M&A
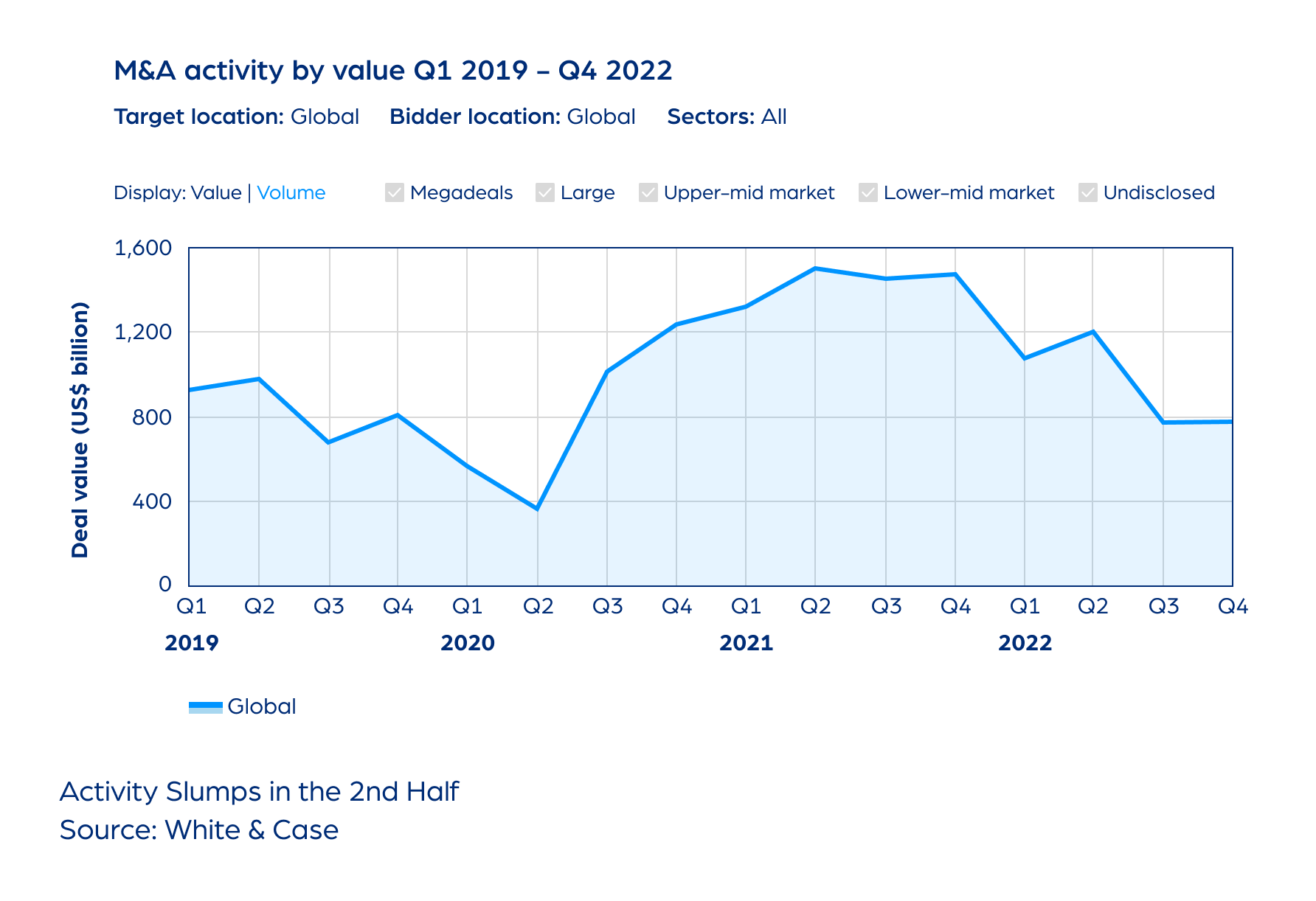
2022: Tough Environment, Resilient Market
-

M&A
Earnouts: Seeking Safety in a Transaction
-

Virtual Data Room
5 Common Fundraising Questions Answered
-
Virtual Data Room
Corporate Repository: Rethinking the Way You Do Business
-

Virtual Data Room
Simplify Your Audit With a Virtual Data Room
current_page_num+2: 3 -




